See How Cerezyme May Help for Gaucher Disease Type 1 and 3
Cerezyme is the only treatment for Gaucher disease type 1 with over 30 years of time-tested and enduring results.
Cerezyme is also the first and only FDA-approved treatment for the non-CNS symptoms of Gaucher disease type 3.
What Is Cerezyme?
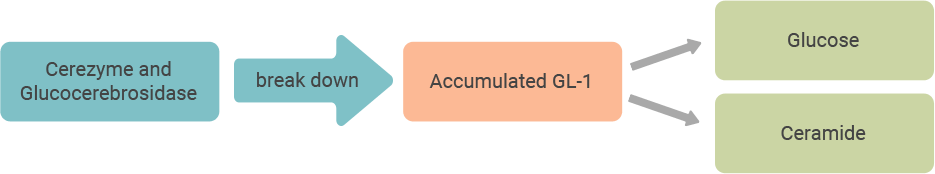
People with Gaucher disease may not have enough of an enzyme called glucocerebrosidase (also known as acid beta glucosidase) that breaks down a fatty substance called glucosylceramide, or GL-1. Cerezyme is a modified form of glucocerebrosidase, and it works to reduce the buildup of GL-1 in the body.
Cerezyme is the longest-standing and trusted treatment for Gaucher disease type 1, having helped thousands of patients across the United States. It is also the first and only FDA-approved treatment for Gaucher disease type 3.
How Cerezyme works
Cerezyme is designed to reduce the build-up of GL-1 in people who do not have enough of the natural enzyme, glucocerebrosidase, to keep up with their bodies’ production of it. Cerezyme acts like the body’s natural enzyme, breaking down GL-1 into its more basic elements, glucose and ceramide, that can be naturally removed from the body.
How Cerezyme has been proven to help
Cerezyme helped adults and children with Gaucher disease type 1 and type 3 by improving key disease symptoms over the long term, including:
ORGAN PROBLEMS
- Enlarged spleen (splenomegaly) and liver (hepatomegaly)
BLOOD PROBLEMS
- Low red blood cell count
- Reduced platelets in the blood
BONE PROBLEMS
- Low bone mineral density (BMD)*
- Bone pain
- Bone crisis**
- Delayed growth
*Bone mineral density, or bone mass, is the amount of minerals in your bones.
**Bone crisis is an episode of severe bone pain that lasts for more than 3 days and may be accompanied with other symptoms, such as fever.
The studies behind Cerezyme
How has Cerezyme been studied?
Cerezyme was studied in clinical trials to show its efficacy and safety in adults and children with Gaucher disease. Gaucher Registry studies support the efficacy and safety findings in the clinical trials.
What is the Gaucher Registry?
The Gaucher Registry is an international database sponsored by Sanofi that was started to keep track of the experiences of people with Gaucher disease. Data from the Registry is used to help researchers and physicians understand the impact of Gaucher disease and the effectiveness of long-term treatment. Since 1991, the Registry has collected voluntary information from nearly 7,000 people with Gaucher disease worldwide.
Gaucher Disease Type 1
To learn more about the Cerezyme key efficacy and safety studies, long-term studies, bone studies, or pediatric study, click "See the Results" below.

Key Efficacy and Safety studies
Explore the 6-month pivotal clinical trial and 48-month bone clinical trial


Gaucher Disease Type 3
To learn more about the Cerezyme long-term study, click "See the Results" below.

Cerezyme can cause serious side effects including:
Allergic Reactions (Including Anaphylaxis) and Infusion-Associated Reactions (IARs):
Signs of an allergic reaction reported during or shortly after an infusion included itching, flushing, hives, swelling under the skin, chest discomfort, shortness of breath, coughing, a bluish discoloration of the skin due to diminished oxygen, rapid heart rate, and low blood pressure.
Signs of an infusion reaction included rash, chills, fatigue, infusion-site burning, infusion-site discomfort, or infusion-site swelling, fever, and high blood pressure. Tell your healthcare professional right away if you experience any reactions. Your healthcare professional may slow or stop the infusion or may lower the next dose. Your healthcare professional may decide to give you antihistamine, anti-fever, and/or steroid medications before your infusions and monitor you for new signs and symptoms of a reaction.
Immune Responses:
Approximately 15% of patients treated and tested to date have developed immune responses (antibodies) to Cerezyme during the first year of therapy. These patients have a higher risk of an allergic reaction. Your healthcare professional may periodically test for the presence of antibodies.
Possible side effects
The following adverse reactions associated with the use of Cerezyme were identified in clinical studies or postmarketing reports. Because some of these reactions were reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a cause-and-effect relationship to drug exposure.
There may be possible side effects with Cerezyme.
REPORTED SIDE EFFECTS in adult and pediatric patients
- Dizziness
- Headache
- Rapid heart rate
- A bluish discoloration of skin due to diminished oxygen (cyanosis)*
- Flushing*
- Low blood pressure (hypotension)*
- High blood pressure (hypertension)*
- Cough*
- Difficulty breathing*
- Pneumonia
- High blood pressure in the lungs (pulmonary hypertension)
- Stomach pain
- Diarrhea
- Nausea
- Vomiting
- Serious allergic reaction (anaphylaxis)*
- Hypersensitivity
- Accumulation of fluid under the skin (angioedema)*
- Itching*
- Rash
- Hives*
- Back pain
- Chest discomfort*
- Chills
- Fatigue
- Infusion-site burning
- Infusion-site discomfort
- Infusion-site swelling
- Fever
*Signs and symptoms suggestive of allergic reaction (hypersensitivity) and other infusion-associated reactions.
CNS=central nervous system; ICGG=International Collaborative Gaucher Group.
|
|
Treating With Cerezyme
|
|
|
Get Support
|
Important Safety Information
WARNING: SEVERE ALLERGIC REACTIONS
Allergic reactions, including severe reactions that may be serious or life-threatening (known as anaphylaxis), have occurred during the early course and after repeated treatment with CEREZYME.
Your healthcare professional should initiate CEREZYME in a healthcare setting with appropriate medical monitoring and support measures. If a severe allergic or anaphylactic reaction occurs, your healthcare professional will immediately stop the infusion and provide appropriate medical treatment.
Seek immediate medical care should symptoms occur.
Cerezyme can cause serious side effects including:
Allergic Reactions (Including Anaphylaxis) and Infusion-Associated Reactions (IARs):
Signs of an allergic reaction reported during or shortly after an infusion included itching, flushing, hives, swelling under the skin, chest discomfort, shortness of breath, coughing, a bluish discoloration of the skin due to diminished oxygen, rapid heart rate, and low blood pressure.
Signs of an infusion reaction included rash, chills, fatigue, infusion-site burning, infusion-site discomfort, or infusion-site swelling, fever, and high blood pressure. Tell your healthcare professional right away if you experience any reactions. Your healthcare professional may slow or stop the infusion or may lower the next dose. Your healthcare professional may decide to give you antihistamine, anti-fever, and/or steroid medications before your infusions and monitor you for new signs and symptoms of a reaction.
Common Side Effects:
- Common side effects reported in adults and children include back pain, chills, dizziness, fatigue, headache, allergic reactions, nausea, fever, and vomiting.
Please see accompanying Full Prescribing Information, including Boxed WARNING.
Cerezyme® (imiglucerase) for injection is indicated for the treatment of non-central nervous system (CNS) manifestations of Type 1 or Type 3 Gaucher disease in adult and pediatric patients.
Important Safety Information
WARNING: SEVERE ALLERGIC REACTIONS
Allergic reactions, including severe reactions that may be serious or life-threatening (known as anaphylaxis), have occurred during the early course and after repeated treatment with CEREZYME.
Your healthcare professional should initiate CEREZYME in a healthcare setting with appropriate medical monitoring and support measures. If a severe allergic or anaphylactic reaction occurs, your healthcare professional will immediately stop the infusion and provide appropriate medical treatment.
Seek immediate medical care should symptoms occur.
Cerezyme can cause serious side effects including:
Allergic Reactions (Including Anaphylaxis) and Infusion-Associated Reactions (IARs):
Signs of an allergic reaction reported during or shortly after an infusion included itching, flushing, hives, swelling under the skin, chest discomfort, shortness of breath, coughing, a bluish discoloration of the skin due to diminished oxygen, rapid heart rate, and low blood pressure.
Signs of an infusion reaction included rash, chills, fatigue, infusion-site burning, infusion-site discomfort, or infusion-site swelling, fever, and high blood pressure. Tell your healthcare professional right away if you experience any reactions. Your healthcare professional may slow or stop the infusion or may lower the next dose. Your healthcare professional may decide to give you antihistamine, anti-fever, and/or steroid medications before your infusions and monitor you for new signs and symptoms of a reaction.
Common Side Effects:
- Common side effects reported in adults and children include back pain, chills, dizziness, fatigue, headache, allergic reactions, nausea, fever, and vomiting.
Please see accompanying Full Prescribing Information, including Boxed WARNING.
Cerezyme® (imiglucerase) for injection is indicated for the treatment of non-central nervous system (CNS) manifestations of Type 1 or Type 3 Gaucher disease in adult and pediatric patients.
Important Safety Information
WARNING: SEVERE ALLERGIC REACTIONS
Allergic reactions, including severe reactions that may be serious or life-threatening (known as anaphylaxis), have occurred during the early course and after repeated treatment with CEREZYME.
Your healthcare professional should initiate CEREZYME in a healthcare setting with appropriate medical monitoring and support measures. If a severe allergic or anaphylactic reaction occurs, your healthcare professional will immediately stop the infusion and provide appropriate medical treatment.
Seek immediate medical care should symptoms occur.
Cerezyme can cause serious side effects including:
Allergic Reactions (Including Anaphylaxis) and Infusion-Associated Reactions (IARs):
Signs of an allergic reaction reported during or shortly after an infusion included itching, flushing, hives, swelling under the skin, chest discomfort, shortness of breath, coughing, a bluish discoloration of the skin due to diminished oxygen, rapid heart rate, and low blood pressure.
Signs of an infusion reaction included rash, chills, fatigue, infusion-site burning, infusion-site discomfort, or infusion-site swelling, fever, and high blood pressure. Tell your healthcare professional right away if you experience any reactions. Your healthcare professional may slow or stop the infusion or may lower the next dose. Your healthcare professional may decide to give you antihistamine, anti-fever, and/or steroid medications before your infusions and monitor you for new signs and symptoms of a reaction.
Common Side Effects:
- Common side effects reported in adults and children include back pain, chills, dizziness, fatigue, headache, allergic reactions, nausea, fever, and vomiting.
Please see accompanying Full Prescribing Information, including Boxed WARNING.
Cerezyme® (imiglucerase) for injection is indicated for the treatment of non-central nervous system (CNS) manifestations of Type 1 or Type 3 Gaucher disease in adult and pediatric patients.